 |
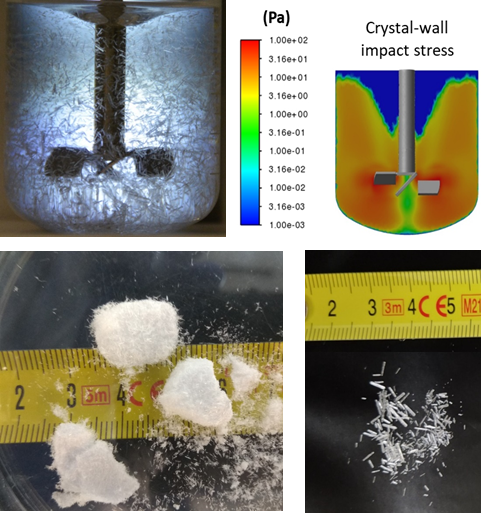
Influence of crystallization process conditions on resulting crystal size distributionThe aim of this project is general characterization of cooled or reactive crystallization processes. To monitor process dynamics we utilize online process analytical probes (i.e., Raman spectroscopy, optical camera, Focused Beam Reflectance method) allowing us to control the process in real time. Offline characterization containing electron or optical microscopy, X-ray diffraction, DSC and other methods are supporting the online process monitoring to confirm final product quality. Using data obtained from the process, we focus on improving the crystal size distribution (CSD) using population balance modelling and CFD to scale-up of the crystallization process. Furthermore, we also include the study of consequent unit operation including filtration, drying, powder mixing or tableting on the drug particle properties (i.e., size, morphology, flow-ability, dissolution properties. 👤 Dominik Martynek |
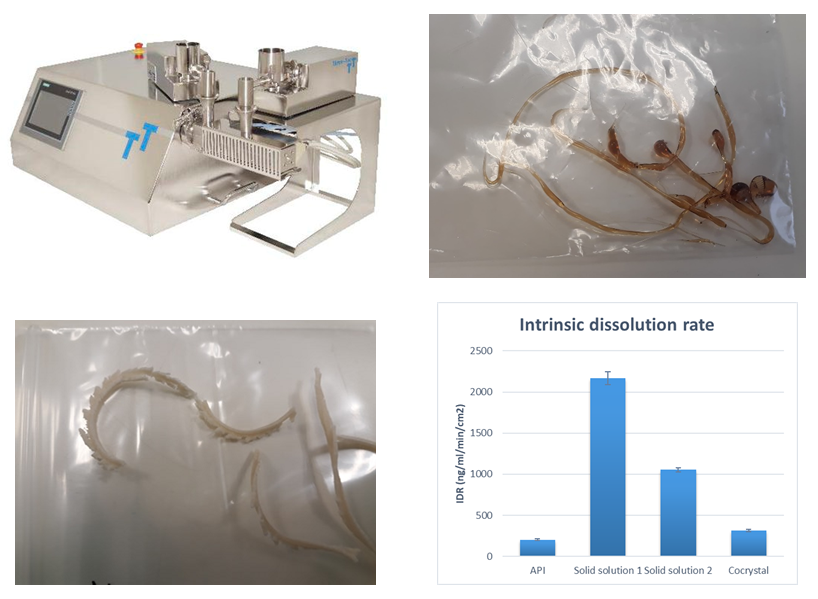 |
Preparation of solid solutions by hot-melt extrusionAmorphous solid solutions represent interesting alternative of drug solid forms with improved solubility. Polymer or coformer screening process is starting with small scale experiments using ball mills or quench cooling method, followed by process scale-up to hot-melt extrusion. Analytical techniques such as XRD, DSC or hot stage microscopy and used to study process of drug amorphization. Once successfully developed we conclude the material development by measurement of dissolution properties and solid solution stability testing. |
Development, scale-up and optimization of preparation processes
: 13.9.2022 16:15, Author: Kristýna Žemlová

